The MIPS eligible clinician is in active engagement with a public health agency to submit immunization data and receive immunization forecasts and histories from the public health immunization registry/immunization information system (IIS).
Measure Overview
Objective: Public Health and Clinical Data Exchange
Measure: The MIPS eligible clinician is in active engagement with a public health agency to submit immunization data and receive immunization forecasts and histories from the public health immunization registry/immunization information system (IIS).
Measure ID: PI_PHCDRR_1
Exclusions: Any MIPS eligible clinician meeting one or more of the following criteria may be excluded from the Immunization Registry Reporting measure if the MIPS eligible clinician:
- Does not administer any immunizations to any of the populations for which data is collected by its jurisdiction’s immunization registry or immunization information system during the performance period. OR
- Operates in a jurisdiction for which no immunization registry or immunization information system is capable of accepting the specific standards required to meet the CEHRT definition at the start of the performance period. OR
- Operates in a jurisdiction where no immunization registry or immunization information system has declared readiness to receive immunization data as of 6 months prior to the start of the performance period.
Exclusion ID(s):
- PI_PHCDRR_1_EX_1
- PI_PHCDRR_1_EX_2
- PI_PHCDRR_1_EX_3
Measure Specifications
Definitions of Terms
Active engagement may be demonstrated in one of the following ways:
Option 1 – Completed Registration to Submit Data: The MIPS eligible clinician registered to submit data with the PHA or, where applicable, the CDR to which the information is being submitted; registration was completed within 60 days after the start of the MIPS performance period; and the MIPS eligible clinician is awaiting an invitation from the PHA or CDR to begin testing and validation. This option allows MIPS eligible clinicians to meet the measure when the PHA or the CDR has limited resources to initiate the testing and validation process. MIPS eligible clinicians that have registered in previous years do not need to submit an additional registration to meet this requirement for each MIPS performance period.
Option 2 – Testing and Validation: The MIPS eligible clinician is in the process of testing and validation of the electronic submission of data. MIPS eligible clinicians must respond to requests from the PHA or, where applicable, the CDR within 30 days; failure to respond twice within a MIPS performance period would result in that MIPS eligible clinician not meeting the measure.
Option 3 – Production: The MIPS eligible clinician has completed testing and validation of the electronic submission and is electronically submitting production data to the PHA or CDR.
Measure Reporting
YES/NO
The MIPS eligible clinician must attest YES to being in active engagement with a PHA to submit immunization data and receive immunization forecasts and histories from the public health immunization registry/immunization information system (IIS).
Scoring Information
- Required for the Promoting Interoperability Performance Category Score: Yes must report on Immunization Registry Reporting and Electronic Case Reporting.
- Measure Score: 10 points
- Eligible for Bonus Score: No
Note: The following measures are included in the Public Health and Clinical Data Exchange objective: for bonus points Public Health Registry Reporting (optional), Clinical Data Registry Reporting (optional), and Syndromic Surveillance Reporting (optional).
In order to earn a score greater than zero for the Promoting Interoperability performance category, MIPS eligible clinicians must:
- Complete the activities required by the Security Risk Analysis and High Priority Practices SAFER Guide, submit their complete numerator and denominator or Yes/No data for all required measures and attest to the Actions to limit or restrict compatibility or interoperability of CEHRT statement.
- Failure to report at least a “1” in all required measures with a numerator or reporting a “No” for a Yes/No response measure (except for the SAFER Guides measure2) will result in a total score of 0 points for the Promoting Interoperability performance category
Freedom | EHR Workflow
MIPS Conformance Calculation
To meet this measure, MIPS eligible clinicians must attest Yes to being in active engagement with an immunization registry to submit immunization data and receive immunization forecasts and histories from the public health immunization registry/immunization information system (IIS).
After completing the requirements for this objective, you can manually attest to the objective and its associated measures from the EHR Dashboard > Right-Click Menu > MIPS Conformance screen.
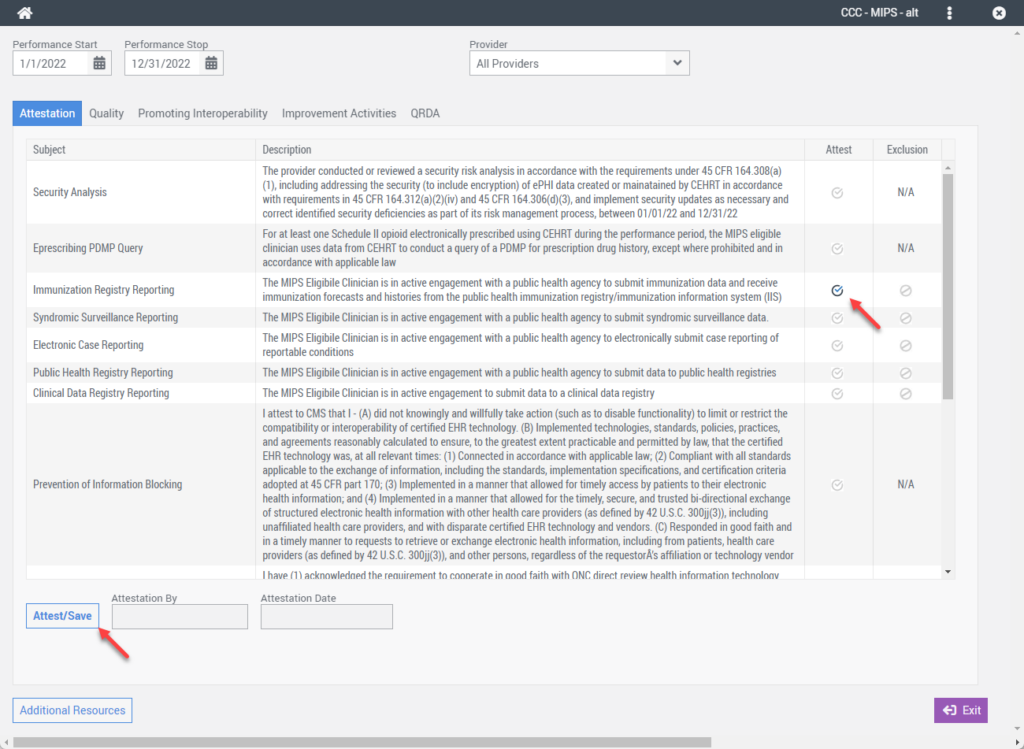
Data Validation
CMS will publish guidance to help clinicians better understand the supporting documentation they should retain when attesting to the MIPS requirements. CMS calls this guidance “MIPS Data Validation Criteria” as it outlines the types of documentation that should be retained to validate the data the provider submits to CMS at the end of the performance period. You must keep documentation for six years subsequent to submission. We suggest reviewing this validation document to ensure you document your work appropriately.
